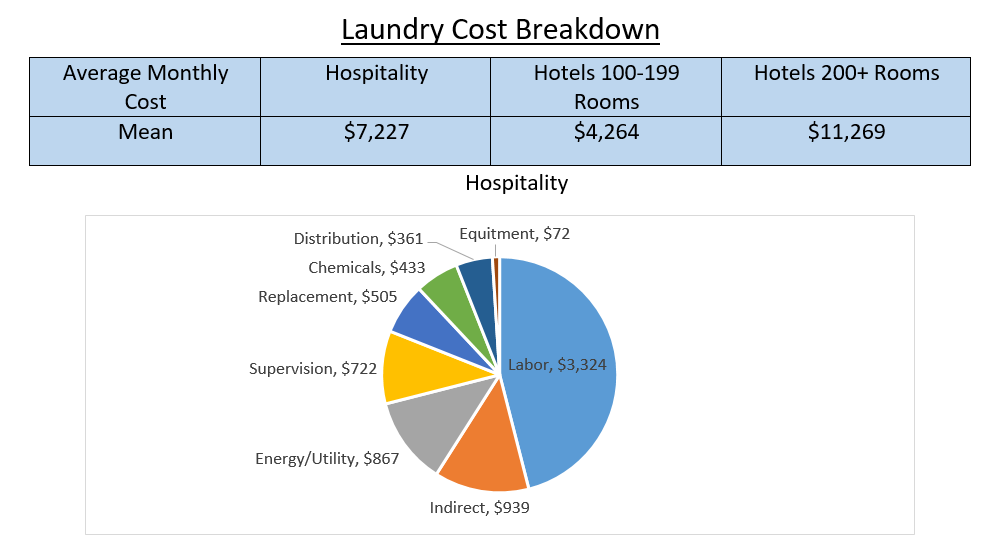
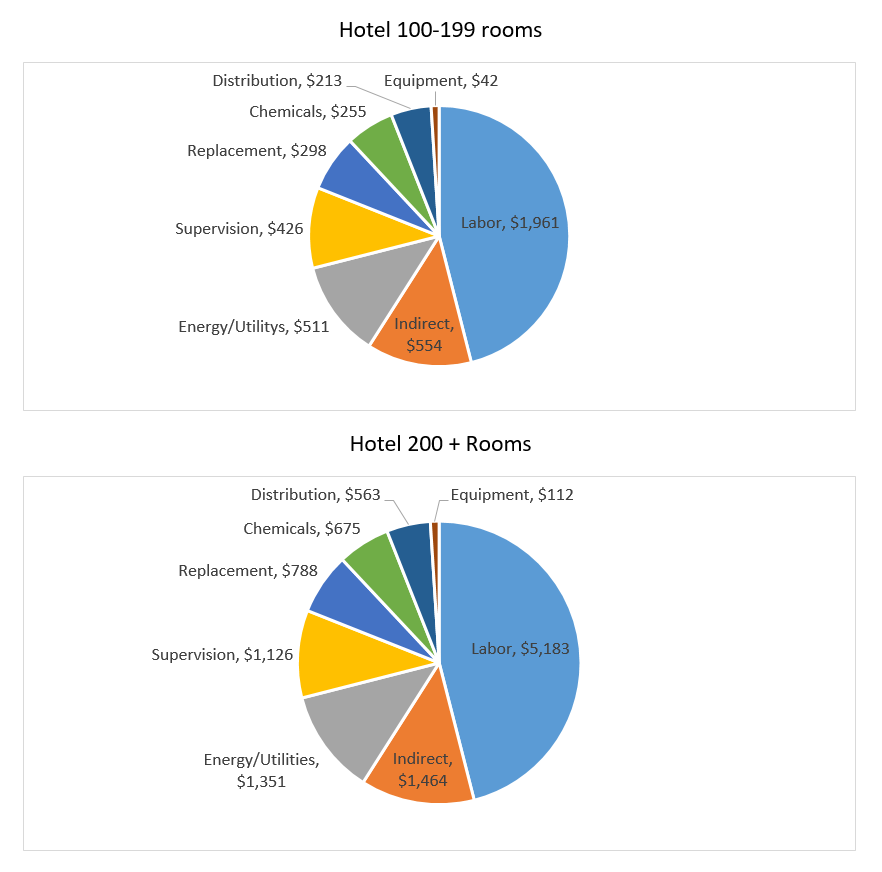
Water Conditions in the Laundry
Water conditions are one of the most important factors when it comes to determining the proper products to use at a laundry account. If you have good water conditions, many products are available for use. If you have poor water conditions, you must first match up the proper products to handle the particular water conditions and then take into consideration other factors such as soil type, soil load, type of linen, and procedures. Here are a few important things to remember about water conditions in the laundry and water impurities that you can check with your total test kit.
Water Hardness – If you have water hardness above 12 GPG you cannot use a Built Detergent. You must use a separate Break and Suds. Water harness is a combination of minerals that will build up on fabric and cause greying. In order to prevent this, you will have to add more water conditioners. Built Detergents have a limited amount of water conditioners. Break products have a high amount of water conditioners. This allows you to be able to adjust the amount of Break going into a laundry machine to treat the water hardness without causing excessive foaming. Water hardness will also reduce the effectiveness of detergents, which may trap soils into the linen.
Bicarbonate alkalinity – If you have bicarbonate alkalinity over 200 ppm you must use a separate Sour and Softener. This way you can increase the amount of Sour that will dissolve the bicarbonate alkalinity without increasing the Softener. This can also waterproof linen if too much is used. Too much bicarbonate alkalinity trapped in the linen will cause some of the same issues that water harness will. It can cause graying of linen and entrap soils in the linen.
Iron – Water containing as little as 0.2 ppm can cause discoloration in linen. Any account with a significant amount of iron in the water should have a separate Sour and Softener. Sour/Soft combination products only effect pH and not iron. Iron in linen can cause a variety of staining discolorations from yellow to reddish brown. Iron can also trap oily soils in the linen making them difficult to remove without removing the iron in the linen. At accounts with high levels of iron in the water supply it is recommended to use an oxygenated bleach instead of a chlorinated bleach. Chlorine will oxidize iron trapped in the linen and cause yellow/brown stains. If you have to use chlorine bleach in an account with high amounts of iron in the water, try to use as little as possible and use specialized detergents such as a solvent detergent to help maintain whiteness.
Chlorine – Many water treatment plants add chlorine to the water for sterilization purposes. Most municipalities maintain a minimum of 0.5 ppm of chlorine in drinking water. Generally this does not cause any issues, but there may be instances where it can. Some water softener resins can be damaged by chlorine levels as low as 0.5 ppm, creating water hardness issues. Also if there is a high amount of chlorine in the water supply it can cause yellowing of the linen or a reaction in the linen with any trapped iron.
These are just a few water impurities that can cause issues in a laundry account, but they are the most common. You can check these water impurities with your total test kit. However, if a more in-depth water analysis is needed, please contact the U S Chemical Training Department.